Termination
After propagation comes termination. Any further addition of the monomer units to the growing chain is here after stopped, and the growth of the polymer chain is arrested.
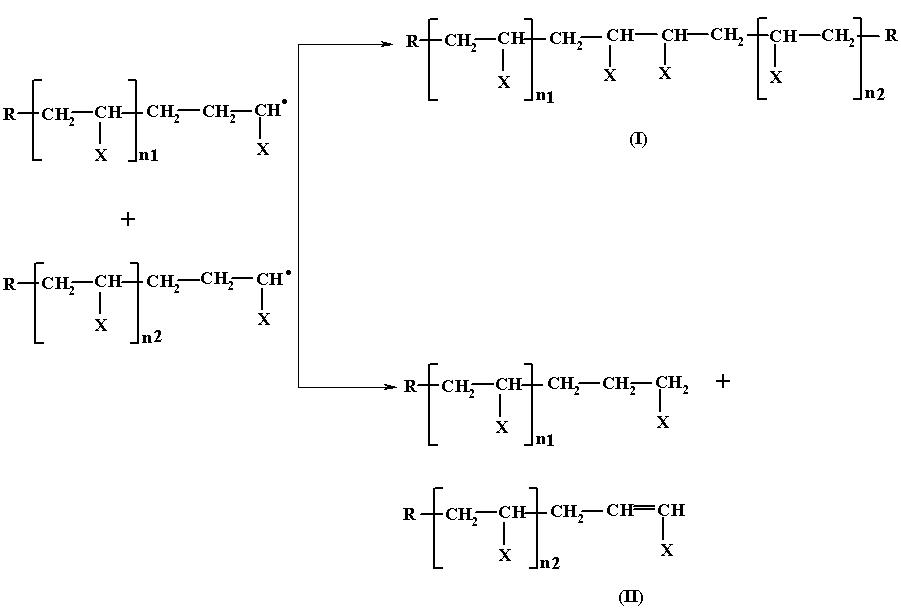
Since the decomposition of the initiator produces many free radicals at the same time each one of them can initiate and propagate the chain growth simultaneously and hence at a given time there may be quite a few growing chains present in the system. Depending on the factors such as temperature, time and monomer and initiator concentration, there exists a statistical probability of the two growing chains coming close to and colliding with each other. When such collision takes place, the following two reactions occur, resulting in the arrest of the chain growth.
In the first case, the two growing chains units by the coupling of the lone pair of electron present in each chain to form an electron pair and thus, nullifying their reactiveness. Since this process involves the coupling of two lone pair of electrons, this kind of termination is known as termination by coupling.
In the second case, one H from one growing chain is abstracted by the other growing chain and utilized for getting stabilized, while the chain which had donated the H, gets stabilized by the formation of a double bond. In this case, the termination process results in the formation of two polymer molecules of shorter chain length as against a single molecule of a longer chain length obtainable by the first method. This type of termination is called termination by disproportination.